
Calixarene-supported Pd–NHC complexes as efficient catalysts for scalable Suzuki–Miyaura cross-couplings - React. Chem. Eng. - X-MOL

Well‐defined PEPPSI‐themed palladium–NHC complexes: synthesis, and catalytic application in the direct arylation of heteroarenes - Kaloğlu - 2020 - Applied Organometallic Chemistry - Wiley Online Library

Sulfination by Using Pd‐PEPPSI Complexes: Studies into Precatalyst Activation, Cationic and Solvent Effects and the Role of Butoxide Base - Sayah - 2013 - Chemistry – A European Journal - Wiley Online Library
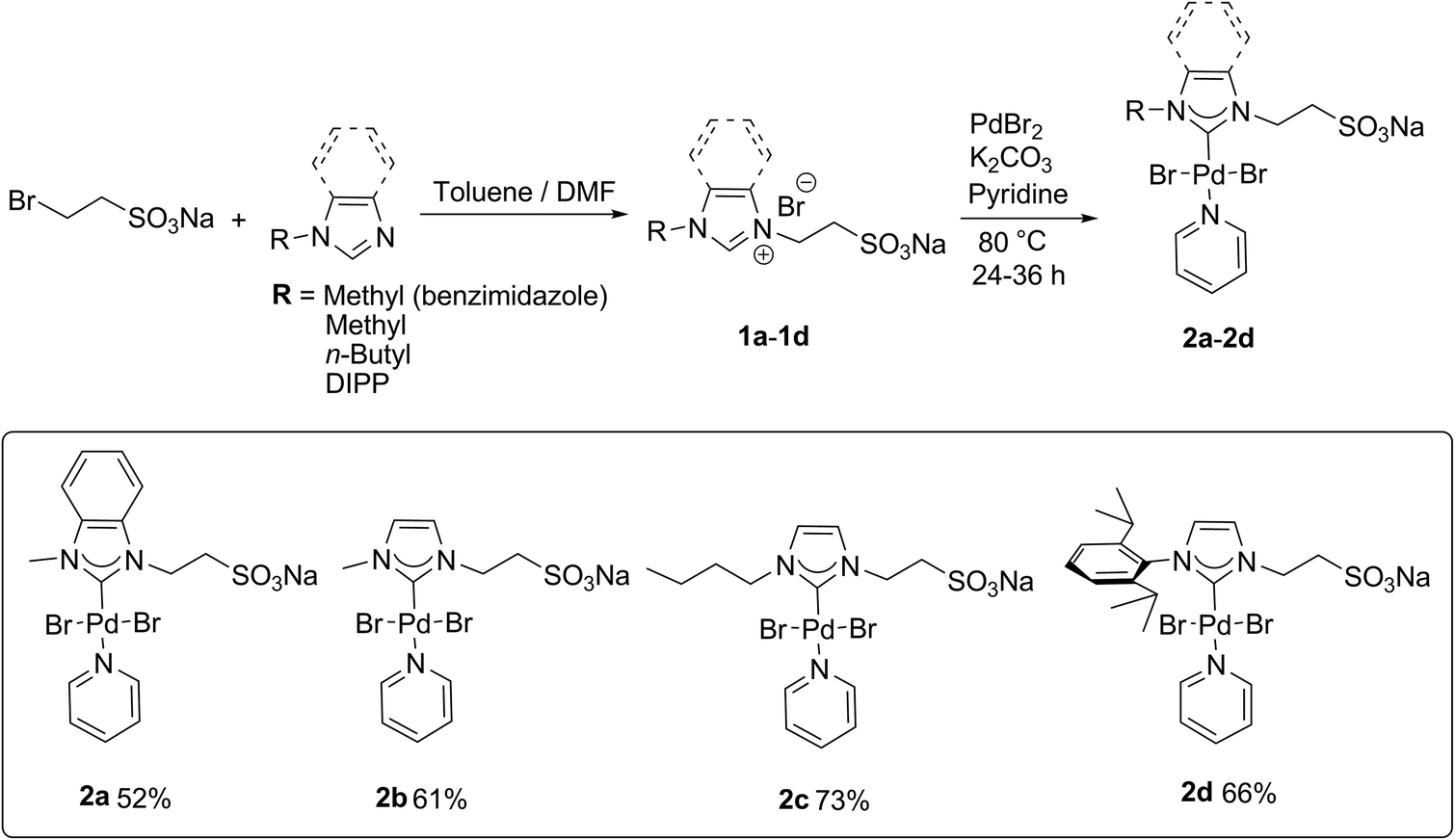
Facile-prepared sulfonated water-soluble PEPPSI-Pd-NHC catalysts for aerobic aqueous Suzuki–Miyaura cross-coupling reactions - Green Chemistry (RSC Publishing) DOI:10.1039/C4GC00986J

PEPPSI‐Type Palladium–NHC Complexes: Synthesis, Characterization, and Catalytic Activity in the Direct C5‐Arylation of 2‐Substituted Thiophene Derivatives with Aryl Halides - Kaloğlu - 2017 - European Journal of Inorganic Chemistry - Wiley Online Library

PDF) Phase-Separable Polyisobutylene Palladium-PEPPSI Precatalysts: Synthesis and Application in Buchwald– Hartwig Amination
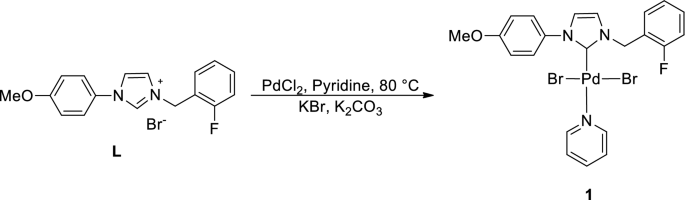
A new PEPPSI type N-heterocyclic carbene palladium(II) complex and its efficiency as a catalyst for Mizoroki-Heck cross-coupling reactions in water | SpringerLink
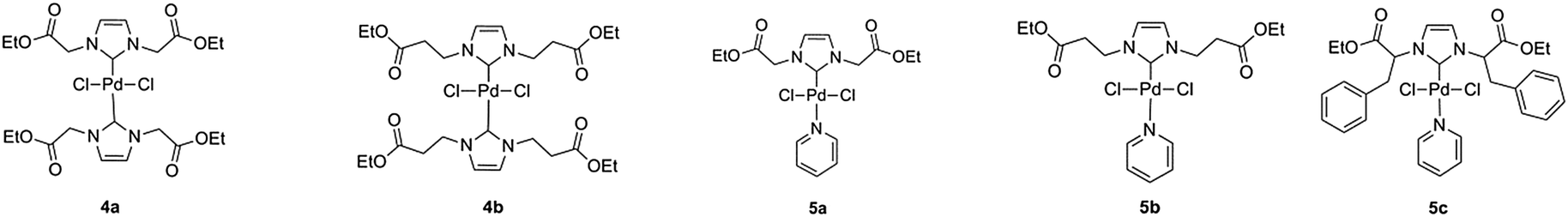
Amino acid-derived N-heterocyclic carbene palladium complexes for aqueous phase Suzuki–Miyaura couplings - New Journal of Chemistry (RSC Publishing)
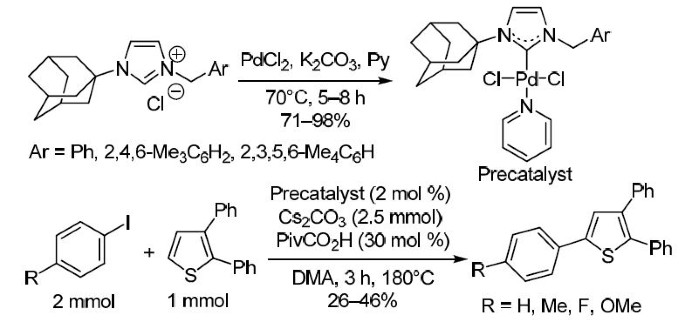
Adamantanyl-substituted PEPPSI-type palladium(II) N-heterocyclic carbene complexes: synthesis and catalytic application for CH activation of substituted thiophenes | SpringerLink

Biaryls Made Easy: PEPPSI and the Kumada–Tamao–Corriu Reaction - Organ - 2007 - Chemistry – A European Journal - Wiley Online Library
![Catalysts | Free Full-Text | Palladium PEPPSI-IPr Complex Supported on a Calix[8]arene: A New Catalyst for Efficient Suzuki–Miyaura Coupling of Aryl Chlorides | HTML Catalysts | Free Full-Text | Palladium PEPPSI-IPr Complex Supported on a Calix[8]arene: A New Catalyst for Efficient Suzuki–Miyaura Coupling of Aryl Chlorides | HTML](https://www.mdpi.com/catalysts/catalysts-10-01081/article_deploy/html/images/catalysts-10-01081-g001.png)
Catalysts | Free Full-Text | Palladium PEPPSI-IPr Complex Supported on a Calix[8]arene: A New Catalyst for Efficient Suzuki–Miyaura Coupling of Aryl Chlorides | HTML
![Catalysts | Free Full-Text | Palladium PEPPSI-IPr Complex Supported on a Calix[8]arene: A New Catalyst for Efficient Suzuki–Miyaura Coupling of Aryl Chlorides | HTML Catalysts | Free Full-Text | Palladium PEPPSI-IPr Complex Supported on a Calix[8]arene: A New Catalyst for Efficient Suzuki–Miyaura Coupling of Aryl Chlorides | HTML](https://www.mdpi.com/catalysts/catalysts-10-01081/article_deploy/html/images/catalysts-10-01081-i002.png)
Catalysts | Free Full-Text | Palladium PEPPSI-IPr Complex Supported on a Calix[8]arene: A New Catalyst for Efficient Suzuki–Miyaura Coupling of Aryl Chlorides | HTML
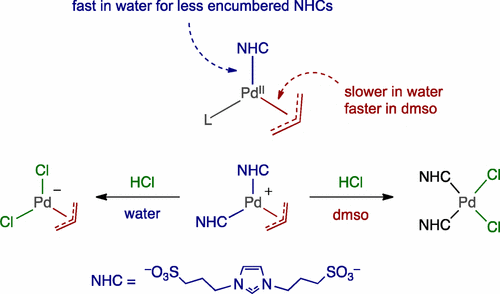
Aqueous-Phase Chemistry of η3-Allylpalladium(II) Complexes with Sulfonated N-Heterocyclic Carbene Ligands: Solvent Effects in the Protolysis of Pd–C Bonds and Suzuki–Miyaura Reactions - Organometallics - X-MOL

Continuous flow Negishi cross-couplings employing silica-supported Pd-PEPPSI–IPr precatalyst - Catalysis Science & Technology (RSC Publishing)

Trimetallic PEPPSI‐Type Palladium N‐Heterocyclic Carbene Complexes – Improved Catalyst Lifetime in the Mizoroki–Heck Coupling Reaction - Martínez‐Olid - 2015 - European Journal of Inorganic Chemistry - Wiley Online Library

Synthesis of bridged palladium-PEPPSI complexes and catalytic studies in C–C cross-coupling reactions - ScienceDirect

PDF) Pd-PEPPSI-IPentCl: A new highly efficient ligand-free and recyclable catalyst system for the synthesis of 2-substituted indoles: Via domino copper-free Sonogashira coupling/cyclization

Investigation of activity, stability, and degradation mechanism of surface-supported Pd-PEPPSI complexes for Suzuki-Miyaura coupling - ScienceDirect
A new PEPPSI type N-heterocyclic carbene palladium(II) complex and its efficiency as a catalyst for Mizoroki-Heck cross-coupling reactions in water | SpringerLink
![Catalysts | Free Full-Text | Palladium PEPPSI-IPr Complex Supported on a Calix[8]arene: A New Catalyst for Efficient Suzuki–Miyaura Coupling of Aryl Chlorides | HTML Catalysts | Free Full-Text | Palladium PEPPSI-IPr Complex Supported on a Calix[8]arene: A New Catalyst for Efficient Suzuki–Miyaura Coupling of Aryl Chlorides | HTML](https://www.mdpi.com/catalysts/catalysts-10-01081/article_deploy/html/images/catalysts-10-01081-i001.png)

![Various [NHC-Pd-PEPPSI] (left) and long alkyl chain-labeled... | Download Scientific Diagram Various [NHC-Pd-PEPPSI] (left) and long alkyl chain-labeled... | Download Scientific Diagram](https://www.researchgate.net/profile/Muhammad_Ghufran_Rafique/publication/317660984/figure/fig1/AS:528262394638337@1502958977918/Various-NHC-Pd-PEPPSI-left-and-long-alkyl-chain-labeled-NHC-Pdallyl-cinnamyl.png)
